is the food code fda a administrative law
The FDA Food Code is based on recommendations from the Conference for Food Protection. Food and drug administration fda publishes the food code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound.
As required by law the Food and Drug Administration publishes regulations in the Federal Register the federal governments official publication for notifying the public of many kinds of agency.
. The FDA is tasked by law to prescribe standards guidelines and regulations with respect to information advertisements. 13 1979 as amended at 54 FR 9035 Mar. Title 1-10110 Food Code.
Subsequently question is who writes the FDA Food Code. A more important skill is the ability to locate the statutes and regulations that comprise food law as well as credible explanations of food laws. Starting in 1996 the Administration labeled nicotine a drug and cigarettes a device used to deliver nicotine and issued regulations governing their format and marketing.
Several tobacco companies sued the FDA to challenge these regulations. B A term that is. These provisions shall be known as the Food Code hereinafter referred to as this Code R9-8-102.
690 on this Website Official. Federal statutes and regulations. Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the retail and food service segment.
Similarly when was the FDA Food Code last updated. The Federal Register and Code of Federal Regulations. It and other federal laws establish the legal framework within.
Where to Find Federal Regulations For a guide to the federal executive-branch regulatory process and the process of conducting federal administrative law research in general see the Law Librarys Administrative Law Research Guide. The Philippines Food and Drug Administration FDA issued Administrative Order No. It represents FDAs best.
44 FR 22348 Apr. Food and Drug Administration The Food Code is a model for safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer. This administrative regulation establishes a uniform code for the regulation of all food service establishments and retail food stores for the purpose of protecting the public health.
Code and the Code of Federal Regulations are the sources of US. The limited availability of material under this paragraph does not constitute prior disclosure to the public as defined in 2081 and no information subject to a particular order is to be submitted to or received or considered by FDA in support. The Administrative Procedure Act license format license suspension and revocation inspection standardization and documentation and cease and desist and abatement.
1 Food additive has the meaning stated in the Federal Food Drug and. It covers the labeling of all prepackaged food products including food. The Federal Food Drug and Cosmetic Act FDC Act is a federal law enacted by Congress.
2014-0030 or otherwise known as the Revised Rules and Regulations Governing the Labeling of Prepackaged Food Products FDA Labeling Regulations. The administrative law judge or other presiding officer may impose other conditions or safeguards. 66 rows FDA Food Code adoptions by States.
Regulations that Govern Food Product Labelling in the Philippines. The Food Drug and Cosmetic Act gave the Food and Drug Administration power to regulate drugs and drug-related devices. Applicability This Article does not apply to the following.
DHS 196 Appendix WISCONSIN ADMINISTRATIVE CODE 822 The Wisconsin Administrative Code on this web site is updated on the 1st day of each month current as of that date. Mandate of the Food and Drug Administration. Volume II of the CFR which is the focus of this article contains an administrative law backgrounder detailing the specifics of administrative law because FDA simultaneously acts as a regulator of federal law a watchdog and a facilitator.
The FDA Food Code is not federal law. The laws administered by the Commissioner or the laws administered by the Food and Drug Administration means all the laws that the Commissioner is authorized to administer. Adoption of the Food Code represents a successful federalstatelocal partnership in improving food safety.
The FDA Food Code is currently updated every four years with the last full update in 2013. It is the FDAs best advice for ways to ensure that food at retail and in foodservice is safe properly protected and presented. The Code of Federal Regulations CFR Title 21 Part 17 and the sections of the Federal Food Drug and Cosmetic Act and the Public Health Service Act cited therein authorize the Food and Drug Administration FDA to impose civil money penalties on individuals or entities based on certain determinations.
3720 or otherwise known as the Food Drug and Cosmetic Act is the law creating the Food and Drug Administration FDA which is under the Office of the Secretary of the Department of Health. B Administrative support for a Board is to be provided only by the office of the Commissioner and the office of the Chief Counsel for FDA. The FDA Food Code is not federal law.
A Part 10 governs practices and procedures for petitions hearings and other administrative proceedings and activities conducted by the Food and Drug Administration under the Federal Food Drug and Cosmetic Act the Public Health Service Act and other laws which the Commissioner of Food and Drugs administers. See also Are the Codes Register June 2013 No. The extensive and evolving nature of food law makes it impossible to know all the law.
The FDA Food Code is not federal law. The 2017 Food Code is the most recent full edition published by FDA. This is the portion of the Code of Federal Regulations that governs food and drugs within the United States for the Food and Drug Administration FDA the Drug Enforcement Administration DEA and the Office of National Drug Control Policy ONDCP.
Food and Drug Administration FDA publishes the Food Code. It is up the agencies that have responsibility for food safety to either adopt or adapt the FDA code to their own jurisdiction. Codified federal regulations on food drugs and cosmetics can be found in Title 21 of the Code of Federal Regulations.

What Is Regulatory Dossier And What Does It Contain The Kolabtree Blog
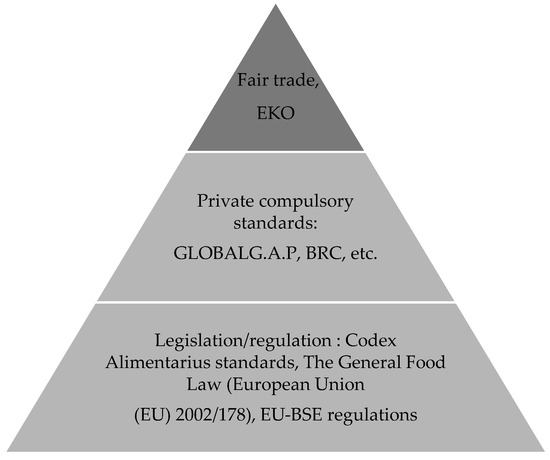
Foods Free Full Text A Review On The Rising Prevalence Of International Standards Threats Or Opportunities For The Agri Food Produce Sector In Developing Countries With A Focus On Examples From The

483 Inspection Observation Responses Customs International Trade Law Firm

Device Advisories Food And Drug Administration

Federal Register Authorization And Revocation Of Emergency Use Of Drugs During The Covid 19 Pandemic Availability
Understanding The Safe Food For Canadians Regulations A Focus On Sanitation Hygiene And Material Handling Requirements Your Partners In Hygiene

Canada Inspections Of Clinical Investigators Socra Blog

Prohibited Claims In Food Product Labels Under Fda Regulations And Philippine Law Law Firm In Metro Manila Philippines Corporate Family Ip Law And Litigation Lawyers

Federal Register Authorization And Revocation Of Emergency Use Of Drugs During The Covid 19 Pandemic Availability
